NHP Licensing
All natural health products (NHPs) sold in Canada require a product licence before being marketed. Obtaining a licence requires submitting to Health Canada detailed information on the product. Once a product licence application has been assessed and granted market authorization by Health Canada, the product label will bear an eight digit product licence number preceded by the distinct letters NPN (Natural Product Number).
The product licence number on the label assures consumers that the product has been reviewed and approved by Health Canada for safety and efficacy.
At PanaCare, we are your Natural Health Product [NHP] regulatory, compliance and labeling experts in Vancouver, Canada.
What products can/should acquire product licence (NPN) before marketing them?
To be legally sold in Canada, all natural health products must have a product licence (8-digit NPN). Product licences (NPN) are required for most products containing the following ingredients (please refer to the list by function claims or ingredient category):
- vitamins/minerals
- herbs or their extracts
- homeopathic medicine
- traditional Chinese medicine
- nutrients (e.g. amino acids, fatty acids, enzymes)
- probiotics
Also, the Canadian sites that manufacture, package, label and import these natural health products must have site licences (GMP certificate). Our manufacturing facility is fully GMP-compliant and granted with site licence by Health Canada.
NPN Application Timeline
- Quote sent within 1~5 working days
- Sign consulting/service contract
- File application within 1 month
- NPN approval, usually within 3~6 month after submission (full refund if not approved within 1 year)
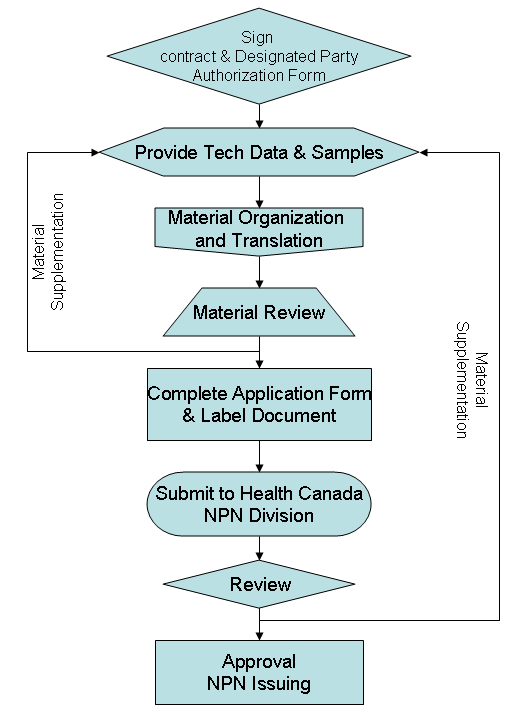
NPN Application Flowchart